An earlier post shows analysis of NIST data on the atomic masses of nuclei: "Binding Energy of Neutrons and Protons".
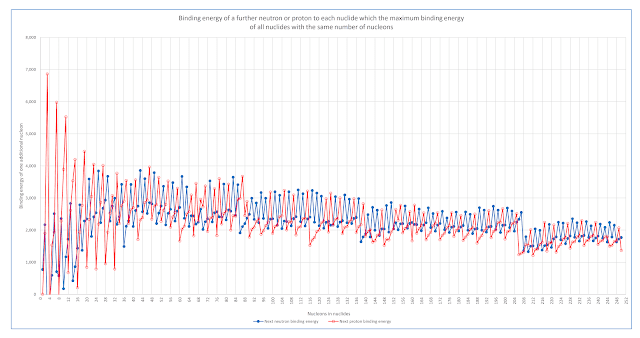
The following chart uses that data to identify the shell structure of nucleons inside atomic nuclei.
The starting point is to find for each nuclide those with the combination of protons and neutrons that have the maximum binding energy. (A nuclide is a nucleus with the same total number of nucleons, no matter how many are protons and how many are neutrons.)
Then the binding energy of adding one further proton or one further neutron to those nuclides is calculated and displayed in the following chart.
Bands with decreasing binding energies are evident for nuclides with between 88 and 140 nucleons, with between 140 and 208 nucleons, and those with more than 208 nucleons.
Inspecting the specific nuclides at the transitions suggests nucleons are arranged in shells in much the way that electrons are distributed in shells around atoms...
- The nuclide with 86 nucleons with the greatest binding energy of all nuclides with 86 nucleons is Krypton-86 which has 36 protons and 50 neutrons in its nucleus. The binding energy of a 51st neutron is markedly lower than the binding energy of all preceding neutrons. This is consistent with the "filling" of an available shell by the 50th neutron, and that further neutrons can only be bound in a shell with lower binding energies available.
- The nuclide with 116 nucleons with the greatest binding energy of all nuclides with 116 nucleons is Tin-116 which has 50 protons and 66 neutrons in its nucleus. The binding energy of a 51st proton is markedly lower than the binding energy of all preceding protons. This is consistent with the "filling" of an available shell by the 50th proton, and that further protons can only be bound in a shell with lower binding energies available.
- The nuclide with 138 nucleons with the greatest binding energy of all nuclides with 138 nucleons is Barium-138 which has 56 protons and 82 neutrons in its nucleus. The binding energy of an 83rd neutron is markedly lower than the binding energy of all preceding neutrons. This is consistent with the "filling" of an available shell by the 82nd neutron, and that further neutrons can only be bound in a shell with lower binding energies available.
- The nuclide with 206 nucleons with the greatest binding energy of all nuclides with 206 nucleons is Lead-206 which has 82 protons and 124 neutrons in its nucleus. The binding energy of an 83rd proton is markedly lower than the binding energy of all preceding protons. This is consistent with the "filling" of an available shell by the 82nd proton, and that further protons can only be bound in a shell with lower binding energies available.
- The nuclide with 208 nucleons with the greatest binding energy of all
nuclides with 208 nucleons is Lead-208 which has 82 protons and 126
neutrons in its nucleus. The binding energy of a 127th neutron
is markedly lower than the binding energy of all preceding neutrons.
This is consistent with the "filling" of an available shell by the 126th neutron, and that further neutrons can only be bound in a shell with lower binding energies available.

0 comments:
Post a Comment