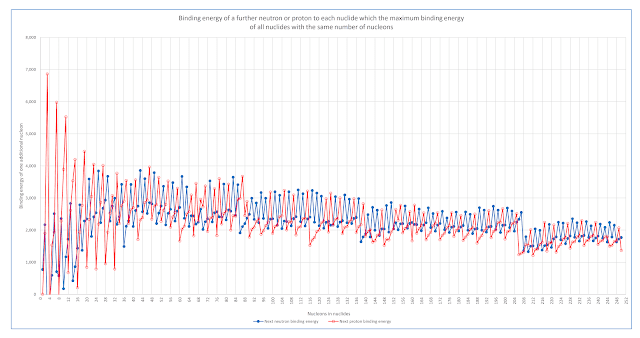
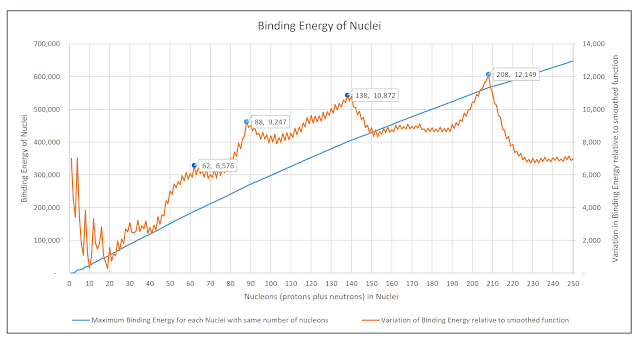
The coal seam gas industry in Queensland has a problem with millions of tonnes of salts that are dissolved in water extracted from coal seams.
In December 2018, Australian Petroleum Production & Exploration Association (APPEA) provided a report to the Queensland Department of Environment and Science titled ‘Queensland Gas: end-to-end water use, supply and management’ (the APPEA Report):
The APPEA Report gives an overview of a number of feasibility studies that have been undertaken by industry operators individually or in collaboration with other industry operators. These feasibility studies examined the viability of the identified options through a range of potential risks and impacts such as environmental, economic, safety, technical, regulatory and social factors.
The report summarises the findings of the following options examined by the studies:
- selective salt recovery
- injection
- ocean outfall
- encapsulation.
In summary, selective salt recovery was determined infeasible due to a lack of suitable technology at a commercial scale, high upfront and lifecycle costs, significant energy consumption requirements and low excess demand in the current market.
The report makes an incorrect assumption about the option for selective salt recovery.
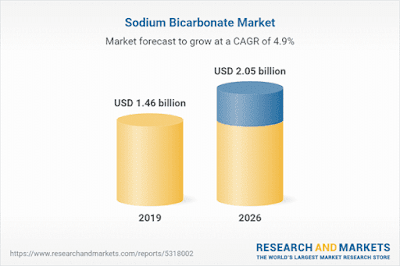 |
| Sodium Bicarbonate Market - Forecasts from 2021 to 2026 |
Available Data
There are several reports on the volume of saline water and the chemicals dissolved in it are available.
The assessment below uses the following quantities selected from reports referenced:
- Total water production: 2,346 gigalitres
(See, at page 14, the University of Queensland Centre for Natural Gas (UQ-CNG) report, "Independent Review: Brine and salt management (Section 6, Queensland Gas: end-to-end water use, supply and management).
- Composition of untreated produced waters from Australian coal basins
Alkalinity
Cl
SO4
Ca
K
Mg
Na
mg/L
1706
593
22.8
10.4
5.6
8.9
1406
(See, at Table 2, "Determining water quality requirements of coal seam gas produced water for sustainable irrigation".)
Opportunity for Selective Salt Recovery
If the quantity for "alkalinity" is ignored for the moment, it can be seen that the total dissolved solids in coal seam gas water are almost entirely sodium and chlorine - making up 1,999 mg per litre of the total.
The volume of produced water (2,346 gigalitres), using that estimate of 1,999 mg per litre, contain 4.69 million tonnes of sodium and chlorine (3.3 million tonnes of sodium and 1.39 million tonnes of chlorine).
The 2018 APPEA report, and a similar report "Coal seam gas ‑ produced water and site management" in August 2014 by the Gas Industry Social and Environmental Research Alliance (GISERA), contain identical phrases describing the dissolved solids in produced water:
"CSG water contains mainly sodium chloride (varying from 200 to more than 10,000 milligrams per litre), sodium bicarbonate [emphasis added] and traces of other compounds."As mentioned above, if the quantity labelled "alkalinity" is ignored, the substance dissolved in produced water is, evidently, almost entirely made up of sodium chloride. That is, common table salt.
Each report on the economic viability of selective salt recovery proceeds on this basis, ignoring the quantity labelled "alkalinity", and concludes with the non sequitur that the recovery of table salt is not commercially viable.
However...
- Sodium carbonate (soda ash) and cooking grade sodium bicarbonate (baking soda) are commercially valuable products.
- The molar ratio of sodium to chlorine in table salt is 1:1.
- The produced water contains 1.39 million tonnes of chlorine. If this was separated with sodium to form table salt, it would use a little under 1 million tonnes of sodium.
- Produced water contains about 3.3 million tonnes of sodium. That is 2.3 million tonnes of sodium more than could be separated as sodium chloride.
There is the opportunity to convert the extra 2.3 million tonnes of sodium dissolved in coal seam gas produced water into sodium bicarbonate (baking soda).
If there is insufficient dissolved carbon dioxide (see "alkalinity", ignored previously) in the produced water to precipitate this quantity of sodium as sodium bicarbonate, the extraction plant could be used for carbon capture - dissolving an external source of carbon dioxide into the produced water - so that all of the available sodium can be extracted as food-grade sodium bicarbonate.
The 2.3 million tonnes of dissolved sodium that is excess to the amount for dissolved sodium chloride is sufficient to manufacture 8.2 million tonnes of sodium bicarbonate or 5.2 million tonnes of sodium carbonate.
The 2.3 million tonnes of sodium chloride dissolved in the coal seam gas produced water could be consumed by making sodium hydroxide and chlorine with it. Solar panels provide low-cost energy, and an innovative plant designed to operate economically during sunlight hours could be a useful innovation: competing with plants that were designed before solar energy costs had reduced to levels that have now been achieved.
There is a large and expanding world market for sodium hydroxide which has uses in many industrial sectors. For instance, sodium hydroxide is used in the purification of the bauxite prior to it being used to make aluminium.
Allied Market Research reports
"The global sodium hydroxide market is anticipated to develop at a momentous growth rate, accredited to growing application of the compound for industrial application."